Nielsen Research Suggests Federal Rescheduling of Cannabis Could Expand Use Fivefold
Study Identifies Medical and Wellness Demand Among U.S. Adults as Cannabis Moves Toward Schedule III
BRUNSWICK , ME, UNITED STATES, January 21, 2026 /EINPresswire.com/ -- Consumer research conducted by Nielsen indicates that federal rescheduling of cannabis could unlock significant unmet demand among U.S. adults, potentially expanding overall participation in cannabis use by over 500%..While current cannabis use encompasses roughly 15% of the U.S. population, according to the 2019 Nielsen U.S. Consumer Research Study, a substantially larger share of adults aged 21 and older report they would consider using cannabis if it were federally legal. When expressed willingness is compared with current participation, the data suggests that legalization and rescheduling—not lack of consumer interest—have been the primary constraints on market growth.
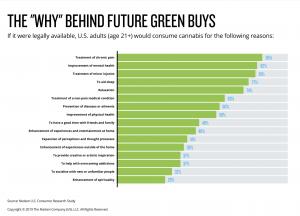
The research indicates that anticipated demand is driven largely by medical and wellness considerations rather than recreational use. Among adults surveyed, the most frequently cited reasons for considering cannabis use if legal included chronic pain management, mental health support, treatment of minor conditions, sleep improvement, and stress reduction.
These findings suggest that cannabis demand aligns closely with established health-adjacent categories such as over-the-counter medications, supplements, and consumer wellness products, positioning cannabis as a mainstream therapeutic option under a regulated framework.
Federal policymakers are currently advancing plans to reschedule cannabis to Schedule III under the Controlled Substances Act, a shift that would allow physician-involved medical use, expanded research, and federally compliant manufacturing and distribution. Industry analysts view the change as a major inflection point that could enable participation by pharmaceutical, healthcare, and consumer products companies that have historically avoided the sector due to federal restrictions.
As the regulatory environment evolves, federally registered manufacturers are expected to play a central role in supplying compliant cannabis materials for medical, research, and wellness markets. Maridose, a DEA-registered bulk manufacturer and importer of cannabis, operates under federal regulatory oversight and is positioned to support Schedule III supply requirements as demand expands.
“The Nielsen data reinforces that consumer interest has been constrained by federal legality, not by lack of demand,” said Richard Shain of Maridose. “As cannabis transitions into Schedule III, the market will increasingly require federally compliant manufacturing and documentation.”
While large pharmaceutical and consumer health companies have monitored consumer demand data in anticipation of regulatory change, much of the existing cannabis industry remains focused on state-level markets. Analysts note that this divergence may create challenges for operators unprepared for federally regulated supply chains as medical and wellness demand increases.
Nielsen’s findings suggest that federal rescheduling represents more than a regulatory adjustment, instead serving as a catalyst for broader participation in cannabis use among U.S. adults as legal barriers are reduced.
Richard Shain
Maridose LLC
Info@maridose.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.