Water for Injection Market Anticipated to Reach USD 71.7 Bn, Expand at a CAGR of 8.0% by 2035 | Analysis Report by TMR
The Global Market for Water for Injection Market Grow at a CAGR of 8.0% by 2035 : Growing Adoption of Single-Use Systems in Pharmaceutical Manufacturing
The Global Market for Water for Injection Market Grow at a CAGR of 8.0% by 2035 : Growing Adoption of Single-Use Systems in Pharmaceutical Manufacturing”
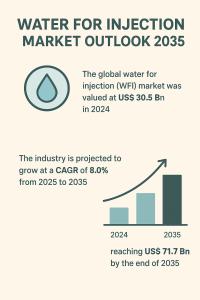
WILMINGTON, DE, UNITED STATES, September 5, 2025 /EINPresswire.com/ -- The global Water for Injection market, a critical component of the pharmaceutical and biotechnology industries, is on a trajectory of significant growth. WFI is an ultra-pure, sterile, and non-pyrogenic form of water essential for the production of injectable pharmaceuticals, vaccines, and biologics. Valued at US$ 30.5 billion in 2024, the market is projected to grow at a robust Compound Annual Growth Rate (CAGR) of 8.0% from 2025 to 2035, reaching an impressive valuation of US$ 71.7 billion by the end of 2035. This expansion is driven by the escalating demand for high-purity water and the stringent regulatory standards governing sterile drug production. The WFI market's role as a cornerstone of modern medicine is becoming more pronounced as the world's population ages and the prevalence of chronic diseases increases, necessitating a steady supply of safe and effective injectable therapies.— Transparency Market Research
Dive Deeper into Data: Get Your In-Depth Sample Now! https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=84885
Key Drivers of Market Growth
Several factors are converging to fuel the growth of the WFI market, each playing a vital role in shaping its future.
1. Rising Demand for Biopharmaceuticals and Injectable Drugs: The global burden of chronic diseases such as cancer, diabetes, and cardiovascular disorders is a major driver. As the demand for injectable medications and biologics—including vaccines, monoclonal antibodies, and gene therapies—continues to grow, so does the need for high-quality WFI. These complex therapies require the highest quality WFI to ensure sterility and efficacy, directly boosting market demand. Furthermore, the rise of biosimilars, which are biological products highly similar to already approved reference biologics, is expanding the market for these therapies and, by extension, for WFI.
2. Stringent Regulatory Compliance: Regulatory bodies like the United States Pharmacopeia (USP), the European Pharmacopoeia (EP), and the World Health Organization (WHO) enforce strict quality control standards for water used in pharmaceutical manufacturing. Adherence to these regulations is non-negotiable for manufacturers. This stringent framework drives continuous investments in advanced WFI production, storage, and monitoring systems to prevent contamination and ensure product safety. The need for validation and continuous monitoring of WFI systems, including real-time testing for total organic carbon (TOC) and endotoxin levels, is a major factor compelling companies to adopt modern, compliant technologies.
3. Technological Advancements in Purification: While multi-effect distillation and vapor compression distillation remain the gold standards due to their reliability, there is a significant shift towards more energy-efficient and cost-effective membrane-based purification technologies. Reverse osmosis (RO) and ultrafiltration (UF) are gaining prominence. Innovations in cold WFI technology, which uses these membrane systems at ambient temperatures, are further lowering operational costs, reducing energy consumption, and increasing adoption, particularly in new manufacturing facilities. The development of advanced membranes and integrated systems is a key trend, offering a more sustainable and economically viable alternative to traditional distillation.
4. Growth in Contract Manufacturing: Pharmaceutical companies are increasingly outsourcing drug production to Contract Development and Manufacturing Organizations (CDMOs). This strategic shift allows them to focus on core activities like research and development while leveraging the specialized expertise and infrastructure of CDMOs. As these organizations expand their capacity to meet bulk production demands for a diverse range of clients, the need for a robust and high-purity WFI supply to support their operations also rises. This trend is particularly strong in emerging markets, where CDMOs are building new, state-of-the-art facilities.
Market Segmentation and Regional Trends
The WFI market is a dynamic landscape segmented by type, application, and region, with distinct trends shaping each segment.
By Type: The market includes various grades of water, with sterile WFI and bacteriostatic WFI being key segments. Sterile WFI, which is pre-sterilized and ready for direct use, held a significant market share, driven by its critical role in injectable drug formulations. Bacteriostatic WFI, which contains an antimicrobial agent, is used primarily for multi-dose formulations. The growing adoption of single-use systems in manufacturing is also a prominent trend, as it reduces the risk of cross-contamination and simplifies cleaning validation processes.
By Application: The primary application segments include pharmaceutical and biotechnology companies, which are the largest consumers, and hospitals, clinics, and research laboratories. WFI is used for a wide range of applications, including as a solvent for reconstituting powdered drugs, a diluent for liquid medications, a component of cell culture media, and for equipment cleaning and sterilization. The formulation of parenteral drugs remains the largest application segment, accounting for a majority of the market share.
By Region: North America continues to hold the largest market share, driven by its well-established pharmaceutical and biotechnology industries, significant investments in R&D, and a stringent and mature regulatory framework. The Asia Pacific region is emerging as the fastest-growing market, with a projected CAGR of over 10%. This growth is fueled by expanding pharmaceutical manufacturing, increasing healthcare investments, rising disposable incomes, and government initiatives aimed at improving healthcare infrastructure, particularly in countries like China and India. The European market also remains a significant contributor, supported by its advanced pharmaceutical sector and adherence to strict regulatory standards.
Challenges and Opportunities
The WFI market's growth is not without its hurdles and presents new opportunities for innovation.
Challenges: The high initial cost of setting up and maintaining WFI production systems can be a significant barrier for smaller players. The complexity of these systems and the need for continuous, strict quality control to prevent contamination during production, storage, and distribution require substantial capital investment and operational expertise. Furthermore, evolving and differing regulatory frameworks across regions present a challenge for global manufacturers, necessitating a flexible and adaptable approach to compliance.
Opportunities: The market offers significant opportunities in the development of specialized systems for continuous flow-through processes, which can enhance efficiency and reduce batch-to-batch variability. The integration of IoT-based sensors and AI-driven monitoring systems for real-time quality control presents a major avenue for innovation, enabling predictive maintenance and enhanced safety. Additionally, the expansion of biopharma manufacturing in emerging economies provides a large, untapped market for companies that can offer cost-effective and compliant WFI solutions. The focus on sustainability is also creating opportunities for companies to develop systems that reduce water wastage and energy consumption, aligning with broader environmental, social, and governance (ESG) goals.
Competitive Landscape
The WFI market is moderately competitive, with a mix of established global companies and specialized niche players. Key players include Veolia Water Technologies, BWT, MECO, Evoqua Water Technologies, and Merck KGaA. These companies are focused on innovating to offer energy-efficient and cost-effective systems that meet the stringent demands of the pharmaceutical industry. Recent developments include Veolia's introduction of the Polaris 2.0 systems, which enhance energy efficiency and compliance, and the expansion of sterile manufacturing lines by CDMOs like WuXi STA in Asia to meet growing demand. Strategic partnerships, mergers, and acquisitions are also a common strategy to expand service offerings and geographical reach. The future of the market will be defined by advancements in technology that balance high purity with operational efficiency and sustainability, ensuring the continued safety and reliability of injectable pharmaceuticals worldwide.
Eurocrit Labs International
Evoqua Water Technologies
Veolia Water Solutions and Technologies
Thermo Fisher Scientific, Inc.
ICU Medical Inc.
B. Braun Melsungen AG
SteriCare Solutions
Danaher Corporation (Cytiva)
Rocky Mountain Biologicals
Veltek Associates, Inc.
Merck KGaA
Corning Incorporated
Future MediSurgico
Pfizer, Inc.
Baxter International Inc.
Explore Latest Research Reports by Transparency Market Research:
Injection Pen Market: https://www.transparencymarketresearch.com/injection-pen-market.html
Paclitaxel Injection Market: https://www.transparencymarketresearch.com/paclitaxel-injection-market.html
Diabetes Injection Pens Market: https://www.transparencymarketresearch.com/diabetes-injection-pens-market.html
Joint Pain Injections Market: https://www.transparencymarketresearch.com/joint-pain-injection-market.html
Self-injection Device Market: https://www.transparencymarketresearch.com/self-injection-device-market.html
Chemical Injection Skid Market: https://www.transparencymarketresearch.com/chemical-injection-skids-market.html
2-shot Injection Molding Market: https://www.transparencymarketresearch.com/2-shot-injection-molding-market.html
Metal and Ceramic Injection Molding Market: https://www.transparencymarketresearch.com/metal-ceramic-injection-molding.html
Injection Molded Plastics Market: https://www.transparencymarketresearch.com/injection-molded-plastics-market.html
Flavored and Functional Water Market: https://www.transparencymarketresearch.com/flavored-functional-water.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
Atil Chaudhari
Transparency Market Research Inc.
+1 518-618-1030
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.